| Specifics |
- Mercury is the only metal which occurs as a liquid at
ordinary temperatures
- Mercury is one of only two metals which occurs naturally in
both its metallic and oxidized state, The other being copper.
- Mercury will form amalgams with almost all metals except Iron
and Aluminum.
- Occurrence in earth’s crust 0.5 ppm. That is 0.5
mg/kilo.
- Melting point is –38.87 C.
- Boiling point is 356.72 C
- Density at 25C 13.534 gr/cubic centimeter (cc)
- Vapor pressure at 25C 2 X 10 –3 mm
- Surface tension 484 dynes/cm
- Electrical resistivity 95.76 microohms/cm
- Does not tarnish in air at normal temperatures, but when
heated near the boiling point will convert to Mercuric Oxide
- It will react slowly with sulfur at normal temperatures to
form Mercuric Sulfide.
- It will react with Nitric Acid and with hot concentrated
Sulfuric Acid.
- It will not react with dilute Hydrochloric Acid, cold
Sulfuric Acid, or Alkalis.
- It will react with ammonia in the presence of oxygen.
- Metallic Mercury can be recovered from solution by the
addition of hydrogen peroxide in the presence of alkali
hydroxides such as Caustic Soda or Lye.
- Mercury can be recovered from solution by cementing with
Copper, Aluminum, Zinc, etc.
- Mercury will react rather violently with Aluminum. Do not use
Mercury in an Aluminum Gold pan or try to store it in an
Aluminum vessel.
|
| Toxicity |
|
 Mercury
like all heavy metals is toxic. It behaves just like other heavy
metals such as Lead, Copper, Arsenic, Zinc, etc. Heavy metals have
the characteristic of not being easily excreted from the body. If
you ingest a large amount of Mercury for example it will stay in
your body for a long time. If you ingest a little Mercury each day
it will accumulate in your body until if, you take no action, it
could produce toxic symptoms such as hair loss etc. Mercury
like all heavy metals is toxic. It behaves just like other heavy
metals such as Lead, Copper, Arsenic, Zinc, etc. Heavy metals have
the characteristic of not being easily excreted from the body. If
you ingest a large amount of Mercury for example it will stay in
your body for a long time. If you ingest a little Mercury each day
it will accumulate in your body until if, you take no action, it
could produce toxic symptoms such as hair loss etc.
 Mercury
has a relatively high vapor pressure, which means that at normal
temperatures if you left a bowl of mercury out in the air a
significant amount would vaporize and would be in the air. If you
continued to breathe this Mercury containing air you certainly
would ingest a significant amount of Mercury. The most dangerous
thing about Mercury is that the lungs readily absorb the vapors.
This you must avoid. So far as I can determine metallic Mercury is
only slowly absorbed through the skin or mucous membranes. In
fact, Mercury in various forms has been used for medical purposes
for 100’s of years. How many of you have used mercurichrome
or merthiolate to disinfect cuts etc or to swab out a sore throat?
There was at one time a much-used medicine called "Blue Pill
or Blue Mass" which was a mixture of metallic Mercury and
honey. Merck Index states " occasional swallowing of Mercury
is without harm". Mercury
has a relatively high vapor pressure, which means that at normal
temperatures if you left a bowl of mercury out in the air a
significant amount would vaporize and would be in the air. If you
continued to breathe this Mercury containing air you certainly
would ingest a significant amount of Mercury. The most dangerous
thing about Mercury is that the lungs readily absorb the vapors.
This you must avoid. So far as I can determine metallic Mercury is
only slowly absorbed through the skin or mucous membranes. In
fact, Mercury in various forms has been used for medical purposes
for 100’s of years. How many of you have used mercurichrome
or merthiolate to disinfect cuts etc or to swab out a sore throat?
There was at one time a much-used medicine called "Blue Pill
or Blue Mass" which was a mixture of metallic Mercury and
honey. Merck Index states " occasional swallowing of Mercury
is without harm".
 I am
not saying that Mercury is not toxic. It definitely is but so is
almost any chemical that you come in contact with; It is just a
matter of amount. There are a few precautions that you must follow
when working with Mercury. These will be discussed in a following
section. What I'm trying to say is that Mercury deserves respect
but not fear. It has taken a very bad rap at the hands of the
do-gooders whose only knowledge of ,or experience with it is that
they once took their temperature with a rectal thermometer. I am
not saying that Mercury is not toxic. It definitely is but so is
almost any chemical that you come in contact with; It is just a
matter of amount. There are a few precautions that you must follow
when working with Mercury. These will be discussed in a following
section. What I'm trying to say is that Mercury deserves respect
but not fear. It has taken a very bad rap at the hands of the
do-gooders whose only knowledge of ,or experience with it is that
they once took their temperature with a rectal thermometer.
 If any
one of you feel that you have a problem with heavy metal
poisoning, hair loss, loose teeth, kidney damage, muscle tremors
etc please have a check for heavy metal intoxication. It’s
simple and inexpensive. If you have a problem there is a treatment
which involves infusing EDTA (ethylene diamine tetracetic acid)
into your blood. This is a treatment known as "chelation"
and it will very effectively remove the heavy metals from your
system. If any
one of you feel that you have a problem with heavy metal
poisoning, hair loss, loose teeth, kidney damage, muscle tremors
etc please have a check for heavy metal intoxication. It’s
simple and inexpensive. If you have a problem there is a treatment
which involves infusing EDTA (ethylene diamine tetracetic acid)
into your blood. This is a treatment known as "chelation"
and it will very effectively remove the heavy metals from your
system.
|
| Precautions |
- When working with Mercury always use latex gloves, it’s
cheap and is good procedure.
- Always store Mercury in tightly closed containers (not
Aluminum).
- Always put a layer of water on top of the Mercury unless
it is charged Mercury.
- Never heat Mercury or amalgam in an enclosed space.
- If you must heat Mercury do it in the outdoors or a
well-ventilated space.
- Always stand upwind of hot mercury. Do not breathe the fumes.
- Do not let Mercury contact Aluminum. It will destroy it.
- Avoid spilling mercury. It is very difficult to clean up.
- Never heat Mercury indoors or in any enclosed space.
- Never try to distill (retort) Mercury in a glass retort.
|
| How to Clean Mercury |
|
 The
term "clean" can mean different things to different
people. It can mean simply Mercury that has no black crud floating
around on top of it, or, it can mean that the Mercury is mirror
bright, silvery without the usual yellow film of mercuric oxide
floating on top. Or, it can mean that the Mercury is pure Mercury
and contains no other metals dissolved in it. The
term "clean" can mean different things to different
people. It can mean simply Mercury that has no black crud floating
around on top of it, or, it can mean that the Mercury is mirror
bright, silvery without the usual yellow film of mercuric oxide
floating on top. Or, it can mean that the Mercury is pure Mercury
and contains no other metals dissolved in it.
 In
order to remove the usual black crud that inevitably floats around
on top of your Mercury is very simple. Get yourself a funnel and a
coffee filter. Take the round filter and fold it in half twice.
Open it so that one side has one layer of paper and the other has
three layers. Put this cone filter in the funnel. Now take a pin
or needle and put a very small hole in the very tip of the paper.
Pour in your cruddy Mercury. The Mercury should pass through the
filter in tiny drops. If it does not pass through, open the hole
just a bit. If it runs through in a steady stream, the hole is too
big and you will have to start over. This method will remove all
of the floating oxides etc and give you Mercury that you can work
with. In
order to remove the usual black crud that inevitably floats around
on top of your Mercury is very simple. Get yourself a funnel and a
coffee filter. Take the round filter and fold it in half twice.
Open it so that one side has one layer of paper and the other has
three layers. Put this cone filter in the funnel. Now take a pin
or needle and put a very small hole in the very tip of the paper.
Pour in your cruddy Mercury. The Mercury should pass through the
filter in tiny drops. If it does not pass through, open the hole
just a bit. If it runs through in a steady stream, the hole is too
big and you will have to start over. This method will remove all
of the floating oxides etc and give you Mercury that you can work
with.
 Second.
If you have Mercury that has other metals amalgamated in it and is
sort of thick with what appears to be "clots" floating
around on it you can filter these amalgam clots by several
methods. The first and easiest is to purchase a syringe (10 ml or
larger) from the drug store. Make a ball of absorbent cotton and
push it into the bottom of the syringe. Push in the plunger to
pack it as tightly as possible. Pour in your Mercury and force it
slowly through the cotton with the syringe plunger. The residue on
the cotton will contain most of the amalgams including Gold.
Probably the best way to recover the amalgamated metals including
gold is to simply take the cotton ball from the syringe and burn
it with a propane torch or other. Be sure you are outside or in a
well ventilated space. Second.
If you have Mercury that has other metals amalgamated in it and is
sort of thick with what appears to be "clots" floating
around on it you can filter these amalgam clots by several
methods. The first and easiest is to purchase a syringe (10 ml or
larger) from the drug store. Make a ball of absorbent cotton and
push it into the bottom of the syringe. Push in the plunger to
pack it as tightly as possible. Pour in your Mercury and force it
slowly through the cotton with the syringe plunger. The residue on
the cotton will contain most of the amalgams including Gold.
Probably the best way to recover the amalgamated metals including
gold is to simply take the cotton ball from the syringe and burn
it with a propane torch or other. Be sure you are outside or in a
well ventilated space.
 A
second way to remove amalgamated materials from Mercury is to
squeeze it through a piece of chamois. Be sure to wear gloves when
using this method. This method is somewhat cleaner than the cotton
filter in that the amalgam usually separates from the chamois and
you eliminate the need to incinerate the cotton. A
second way to remove amalgamated materials from Mercury is to
squeeze it through a piece of chamois. Be sure to wear gloves when
using this method. This method is somewhat cleaner than the cotton
filter in that the amalgam usually separates from the chamois and
you eliminate the need to incinerate the cotton.
 Remember,
although you have filtered your Mercury, you still have metals in
the Mercury, which are in true solution and that cannot be removed
by filtration. In order to remove all the dissolved/amalgamated
metals from Mercury you will have to retort or distill it. In
order to distill Mercury you should purchase a Mercury retort.
They are relatively inexpensive. Yes, you can make one from
pipefitting etc, but they are usually rather clumsy, massive
affairs, which are a bit of a grunt to use. They’re two types
of Mercury retorts. Vented and non-vented. The non-vented type is
simply a boiling vessel and a cooling tube from which the Mercury
drips into a catch vessel. With this type of retort NEVER
put the exit end under water in a catch vessel. If you do, a very
slight drop in the temperature of the boiling Mercury will create
a vacuum sufficient to suck water back through the system right
into the boiling Mercury at 675 F. Please believe me when I tell
you, that is BAAAAD! It will ruin your whole day and probably put
you in the hospital (if you are lucky). Remember,
although you have filtered your Mercury, you still have metals in
the Mercury, which are in true solution and that cannot be removed
by filtration. In order to remove all the dissolved/amalgamated
metals from Mercury you will have to retort or distill it. In
order to distill Mercury you should purchase a Mercury retort.
They are relatively inexpensive. Yes, you can make one from
pipefitting etc, but they are usually rather clumsy, massive
affairs, which are a bit of a grunt to use. They’re two types
of Mercury retorts. Vented and non-vented. The non-vented type is
simply a boiling vessel and a cooling tube from which the Mercury
drips into a catch vessel. With this type of retort NEVER
put the exit end under water in a catch vessel. If you do, a very
slight drop in the temperature of the boiling Mercury will create
a vacuum sufficient to suck water back through the system right
into the boiling Mercury at 675 F. Please believe me when I tell
you, that is BAAAAD! It will ruin your whole day and probably put
you in the hospital (if you are lucky).
 The
vented type of retort is made specifically to prevent this
problem. In this type, near the exit end of the retort there is a
very small tube, which extends upwards a few inches. The purpose
of this tube is to allow you to immerse the exit end of the retort
in water. If your heat source should fail and the temperature in
the "hot vessel" drop, air will be sucked in through the
small tube instead of water through the exit. In any case, If you
retort your Mercury you will now have Mercury, which is, for all
practical purposes, clean and pure. Any amalgamated metals such as
gold will be left behind in the retort. Actually, in order to have
Mercury which is considered chemically pure it must be distilled
three times. Triple distilled Mercury. This has no practical value
for mining. The
vented type of retort is made specifically to prevent this
problem. In this type, near the exit end of the retort there is a
very small tube, which extends upwards a few inches. The purpose
of this tube is to allow you to immerse the exit end of the retort
in water. If your heat source should fail and the temperature in
the "hot vessel" drop, air will be sucked in through the
small tube instead of water through the exit. In any case, If you
retort your Mercury you will now have Mercury, which is, for all
practical purposes, clean and pure. Any amalgamated metals such as
gold will be left behind in the retort. Actually, in order to have
Mercury which is considered chemically pure it must be distilled
three times. Triple distilled Mercury. This has no practical value
for mining.
|
| Applying Mercury to
Copper Plates |
|
 Every
time I see someone trying to apply Mercury to a copper or brass
plate it makes me react just like they were scraping their
fingernails on a blackboard. I see people spending hours trying to
clean the plate by sanding, scrubbing with steel wool, washing
with acid, etc. Then, worst of all, trying to put metallic Mercury
directly onto the plate by chasing it all around with rags,
sponges, squeegees, and any number of other devices. Of course,
most of the Mercury ends up in their shoes, in the water, on the
ground, everywhere except on the plate. Gentlemen, this is NOT the
way to do it.The simple, effective, professional way to coat one
metal with another is to apply a solution of metal salt onto the
other metal and let the resulting battery action reduce the salt
to metal which will then coat the base metal with a even, thin
film. Every
time I see someone trying to apply Mercury to a copper or brass
plate it makes me react just like they were scraping their
fingernails on a blackboard. I see people spending hours trying to
clean the plate by sanding, scrubbing with steel wool, washing
with acid, etc. Then, worst of all, trying to put metallic Mercury
directly onto the plate by chasing it all around with rags,
sponges, squeegees, and any number of other devices. Of course,
most of the Mercury ends up in their shoes, in the water, on the
ground, everywhere except on the plate. Gentlemen, this is NOT the
way to do it.The simple, effective, professional way to coat one
metal with another is to apply a solution of metal salt onto the
other metal and let the resulting battery action reduce the salt
to metal which will then coat the base metal with a even, thin
film.
 If this
seems complicated, its because I sort of set you up. It’s so
simple as to make your old method seem like building a H bomb. All
you need is a little nitric acid and some Mercury. Dilute your
nitric acid with an equal volume of water; put it in a plastic
bottle with a good tight top. Now put a small glob of Mercury in
the bottle and let it stand for awhile. When you are ready to
apply the Mercury to a plate simply be sure there is no grease on
the plate by wiping with a detergent solution. No matter how
cruddy the plate looks, not to worry, simply dip a swab into the
acid solution and wipe it onto the plate. MAGIC. Your plate is now
coated with Mercury. A bottle/swab such as shoe polish comes in
works nicely for this. If this
seems complicated, its because I sort of set you up. It’s so
simple as to make your old method seem like building a H bomb. All
you need is a little nitric acid and some Mercury. Dilute your
nitric acid with an equal volume of water; put it in a plastic
bottle with a good tight top. Now put a small glob of Mercury in
the bottle and let it stand for awhile. When you are ready to
apply the Mercury to a plate simply be sure there is no grease on
the plate by wiping with a detergent solution. No matter how
cruddy the plate looks, not to worry, simply dip a swab into the
acid solution and wipe it onto the plate. MAGIC. Your plate is now
coated with Mercury. A bottle/swab such as shoe polish comes in
works nicely for this.
 I
think that most folks believe they should have a thick coat of
Mercury on the plate. Actually, the opposite is true. You should
remove excess Mercury with a squeegee or other because as gold
sticks to the plate the mercury film gets thicker and thicker.
When this happens, gravel, which is usually moving over the plate,
will scrub the excess off the plate and you will lose mercury and
any gold that it contains. Erosion of thick amalgam layers from
sluice plate is a common problem and one that usually goes
unnoticed. I
think that most folks believe they should have a thick coat of
Mercury on the plate. Actually, the opposite is true. You should
remove excess Mercury with a squeegee or other because as gold
sticks to the plate the mercury film gets thicker and thicker.
When this happens, gravel, which is usually moving over the plate,
will scrub the excess off the plate and you will lose mercury and
any gold that it contains. Erosion of thick amalgam layers from
sluice plate is a common problem and one that usually goes
unnoticed.
|
| How Amalgamation
Works |
|
 I get a
distinct idea that most folks have a distorted idea of just how
amalgamation works. First of all, the way we in the gold business
talk about amalgam is a bit of a misnomer. A true amalgam is when
one metal is actually dissolved in another in which case we would
not be able to filter out the gold from our amalgamated
concentrates. It would simply pass right through the filter. We
normally utilize the unique properties of partially
amalgamated gold in order to recover it easily. I get a
distinct idea that most folks have a distorted idea of just how
amalgamation works. First of all, the way we in the gold business
talk about amalgam is a bit of a misnomer. A true amalgam is when
one metal is actually dissolved in another in which case we would
not be able to filter out the gold from our amalgamated
concentrates. It would simply pass right through the filter. We
normally utilize the unique properties of partially
amalgamated gold in order to recover it easily.
 Visualize
a gold particle like a golf ball. When it comes in contact with
mercury the mercury begins to dissolve in the gold. Now we have a
gold particle with a layer of mercury sticking to its surface
because of the very high surface tension of mercury. The mercury
will now continue to dissolve in the gold and penetrate deeper
into the particle. This process, however, is rather slow and the
deeper it penetrates the slower it goes. Yes, if you have enough
mercury and enough time the gold will eventually dissolve into the
mercury (or vice/versa). However, in our theoretical particle what
we now have is a center of gold/no mercury, a surface of
gold/mercury, and on top of that a layer of mercury/no gold.
Right, we have our amalgamated gold and now we want to get rid of
the mercury. We just need to heat it, right. Yeah, but look what
happens. As we heat it the excess mercury coating boils off. Now
the true amalgam at the surface gets hot and the mercury boils off
leaving the gold, right? Dead wrong! What happens is as the
mercury evaporates from this surface area the gold which was
dissolved in it falls away from the parent particle and is left as
a usually black powder which you normally throw away because it
don’t look like gold. This same effect is true if you use
nitric acid to remove the mercury. Visualize
a gold particle like a golf ball. When it comes in contact with
mercury the mercury begins to dissolve in the gold. Now we have a
gold particle with a layer of mercury sticking to its surface
because of the very high surface tension of mercury. The mercury
will now continue to dissolve in the gold and penetrate deeper
into the particle. This process, however, is rather slow and the
deeper it penetrates the slower it goes. Yes, if you have enough
mercury and enough time the gold will eventually dissolve into the
mercury (or vice/versa). However, in our theoretical particle what
we now have is a center of gold/no mercury, a surface of
gold/mercury, and on top of that a layer of mercury/no gold.
Right, we have our amalgamated gold and now we want to get rid of
the mercury. We just need to heat it, right. Yeah, but look what
happens. As we heat it the excess mercury coating boils off. Now
the true amalgam at the surface gets hot and the mercury boils off
leaving the gold, right? Dead wrong! What happens is as the
mercury evaporates from this surface area the gold which was
dissolved in it falls away from the parent particle and is left as
a usually black powder which you normally throw away because it
don’t look like gold. This same effect is true if you use
nitric acid to remove the mercury.
 So, the
fact is that every time you amalgamate gold particles and recover
the gold the particles get a little smaller. If you have any
doubts try it. Take some rather fine gold and amalgamate and
recover it several times. After three or four times you will
notice that the particles get smaller and smaller. If you continue
this process, eventually you will end up with very fine, black,
gold powder and no yellow particles. Of course the finer the gold
that you amalgamate the more you will convert to the black powder
form. This is because the finer the gold particles, the more
surface area that is exposed for amalgamation and the larger the
percentage of conversion (or loss). So, the
fact is that every time you amalgamate gold particles and recover
the gold the particles get a little smaller. If you have any
doubts try it. Take some rather fine gold and amalgamate and
recover it several times. After three or four times you will
notice that the particles get smaller and smaller. If you continue
this process, eventually you will end up with very fine, black,
gold powder and no yellow particles. Of course the finer the gold
that you amalgamate the more you will convert to the black powder
form. This is because the finer the gold particles, the more
surface area that is exposed for amalgamation and the larger the
percentage of conversion (or loss).
|
| Recovering Mercury
from Solution |
|
 I’m
sure that all of you who use mercury for catching or cleaning up
concentrates also occasionally use nitric acid also. This means
that you surely will end up with nitric acid solutions, which
contain mercury. Please let me encourage to not throw this
solution away. O.K. I’m the same. I don’t have time or
inclination to spend the time to recover a couple of grams of
mercury. What I do is that I have a "stock pot". A
plastic jug in which I put any leftover mining chemicals. No
matter what, acid, caustic, mercury solutions, anything that even
might have something worth recovering or things that I don’t
want to pour down the sink. When I feel like it I recover the
mercury by cementation usually with a copper strip suspended in
the waste. Assuming that the waste solution is not too acidic, the
mercury will drip to the bottom ready for use again. You can also
use aluminum. You can drop in a little table salt and a white
cloud of silver chloride will settle out. Filter this off and
store it for later silver recovery. If you think there might be
some gold in the solution you can filter it through a coffee
filter add some powered zinc, mossy zinc, or just chunks of zinc.
Any precipitate you get might contain gold. I’m sure most of
you have your own pet methods and that’s fine, just don’t
throw all that stuff away. Keep it for when the weather has you
housebound. I’m
sure that all of you who use mercury for catching or cleaning up
concentrates also occasionally use nitric acid also. This means
that you surely will end up with nitric acid solutions, which
contain mercury. Please let me encourage to not throw this
solution away. O.K. I’m the same. I don’t have time or
inclination to spend the time to recover a couple of grams of
mercury. What I do is that I have a "stock pot". A
plastic jug in which I put any leftover mining chemicals. No
matter what, acid, caustic, mercury solutions, anything that even
might have something worth recovering or things that I don’t
want to pour down the sink. When I feel like it I recover the
mercury by cementation usually with a copper strip suspended in
the waste. Assuming that the waste solution is not too acidic, the
mercury will drip to the bottom ready for use again. You can also
use aluminum. You can drop in a little table salt and a white
cloud of silver chloride will settle out. Filter this off and
store it for later silver recovery. If you think there might be
some gold in the solution you can filter it through a coffee
filter add some powered zinc, mossy zinc, or just chunks of zinc.
Any precipitate you get might contain gold. I’m sure most of
you have your own pet methods and that’s fine, just don’t
throw all that stuff away. Keep it for when the weather has you
housebound.
|
| Charged Mercury |
|
 Now we
have arrived at the mystery of mercury. A lot of folks
have heard of it. Most haven’t. Most that have heard of it
respond " oh yeah, that’s the stuff that company X sells
(for a lot of $’s). It’s some mysterious stuff that you
can’t get anywhere else". Baloney! Merlin the magician
died a long time ago and there just ain’t been no magic
since. You can make all you want right in your carport and it
shouldn’t cost you more than $5.00 tops. Now we
have arrived at the mystery of mercury. A lot of folks
have heard of it. Most haven’t. Most that have heard of it
respond " oh yeah, that’s the stuff that company X sells
(for a lot of $’s). It’s some mysterious stuff that you
can’t get anywhere else". Baloney! Merlin the magician
died a long time ago and there just ain’t been no magic
since. You can make all you want right in your carport and it
shouldn’t cost you more than $5.00 tops.
 First
thing is that I don’t like the term "charged mercury"
but since I can’t think of a better one, we will use it. Now
we all understand amalgamation, right? We also understand that
gold is not the only metal that will form amalgams with mercury.
Mercury will form an amalgam with two other metals of interest.
Sodium or potassium. Doesn’t matter which, they are very
similar and for our purposes it doesn’t matter which you use.
"Charged mercury" is nothing more than a mercury/sodium
amalgam. The trick is how do we make it? O.K., you could just take
some sodium metal and drop little chunks of into hot mercury.
However, there are two things wrong with that procedure. First,
dumping anything into hot mercury is a little hazardous since it
tends to make little mini-explosions. Second, sodium is a metal,
white, very soft, can cut it with a knife, it would be a little
hard to lay your hands on. It’s not a common material. Also,
you would have to store it under oil or kerosene because water
vapor in the air will cause it to burn. It reacts violently
with water and can cause explosions. Not to worry, there is a
better way. There are many ways to skin a cat. The problem is that
first you must catch the cat. We got him! Actually, a
gentleman/scientist named Faraday caught the cat for us. If I can
paraphrase Faradays Law it states in effect that for every
28.6 ampere/ hrs of current you can deposit 1 mole of metal (in
the case of sodium, 23 grams) from solution onto the cathode
of an electrolytic cell. Well that certainly is impressive.
So, what does it mean to us? It gives us a way to produce all the
sodium amalgam that we want cheaply and easily. This will be
discussed in detail later. What can this "magic mercury"
do for us? Sodium amalgam is one of the strongest reducing agents
known to science. If you take a metal oxide such as common rust
(iron oxide) and you "reduce" it you will end up with
metallic iron and oxygen. Sodium amalgam will cause this reaction
to occur. It is the absolute best rust remover that you could ever
devise. It will also reduce other materials such as zinc,
magnesium, manganese, sulfides, etc. This ‘charged mercury"
or mercury amalgam will always be mirror bright and shiny with no
yellow film of mercuric oxide floating on the surface. In order
for mercury to amalgamate with gold the two metals must be able to
come in contact with each other. If either the mercury or the gold
has a coat of anything on it you will never get it to amalgamate.
It’s like the gold is enclosed in little plastic bags. They
just can’t get together. Now the mercury surface is perfectly
clean due to the reaction of the sodium with the water. When this
stuff touches a particle of gold which has it’s own coat of
iron or other metallic oxide or sulfide, it will immediately
reduce that too, leaving only the fine powder metal that will wash
away leaving a nice clean gold surface just waiting to be
amalgamated by the mercury which is also present. One thing that
the amalgam will not remove is oil/grease. For that you will have
to use a detergent. When you get some of this stuff and put it in
water you will note that it fizzes giving off hydrogen gas. That
is why it works. As we said before, sodium reacts with water. What
we have done is to make a sodium battery. When the fizzing stops
it means that all the sodium has reacted and it is now "discharged"
to ordinary mercury. Now you must re-charge it. First
thing is that I don’t like the term "charged mercury"
but since I can’t think of a better one, we will use it. Now
we all understand amalgamation, right? We also understand that
gold is not the only metal that will form amalgams with mercury.
Mercury will form an amalgam with two other metals of interest.
Sodium or potassium. Doesn’t matter which, they are very
similar and for our purposes it doesn’t matter which you use.
"Charged mercury" is nothing more than a mercury/sodium
amalgam. The trick is how do we make it? O.K., you could just take
some sodium metal and drop little chunks of into hot mercury.
However, there are two things wrong with that procedure. First,
dumping anything into hot mercury is a little hazardous since it
tends to make little mini-explosions. Second, sodium is a metal,
white, very soft, can cut it with a knife, it would be a little
hard to lay your hands on. It’s not a common material. Also,
you would have to store it under oil or kerosene because water
vapor in the air will cause it to burn. It reacts violently
with water and can cause explosions. Not to worry, there is a
better way. There are many ways to skin a cat. The problem is that
first you must catch the cat. We got him! Actually, a
gentleman/scientist named Faraday caught the cat for us. If I can
paraphrase Faradays Law it states in effect that for every
28.6 ampere/ hrs of current you can deposit 1 mole of metal (in
the case of sodium, 23 grams) from solution onto the cathode
of an electrolytic cell. Well that certainly is impressive.
So, what does it mean to us? It gives us a way to produce all the
sodium amalgam that we want cheaply and easily. This will be
discussed in detail later. What can this "magic mercury"
do for us? Sodium amalgam is one of the strongest reducing agents
known to science. If you take a metal oxide such as common rust
(iron oxide) and you "reduce" it you will end up with
metallic iron and oxygen. Sodium amalgam will cause this reaction
to occur. It is the absolute best rust remover that you could ever
devise. It will also reduce other materials such as zinc,
magnesium, manganese, sulfides, etc. This ‘charged mercury"
or mercury amalgam will always be mirror bright and shiny with no
yellow film of mercuric oxide floating on the surface. In order
for mercury to amalgamate with gold the two metals must be able to
come in contact with each other. If either the mercury or the gold
has a coat of anything on it you will never get it to amalgamate.
It’s like the gold is enclosed in little plastic bags. They
just can’t get together. Now the mercury surface is perfectly
clean due to the reaction of the sodium with the water. When this
stuff touches a particle of gold which has it’s own coat of
iron or other metallic oxide or sulfide, it will immediately
reduce that too, leaving only the fine powder metal that will wash
away leaving a nice clean gold surface just waiting to be
amalgamated by the mercury which is also present. One thing that
the amalgam will not remove is oil/grease. For that you will have
to use a detergent. When you get some of this stuff and put it in
water you will note that it fizzes giving off hydrogen gas. That
is why it works. As we said before, sodium reacts with water. What
we have done is to make a sodium battery. When the fizzing stops
it means that all the sodium has reacted and it is now "discharged"
to ordinary mercury. Now you must re-charge it.
|
| "Charging "
Mercury |
|
 Charging
mercury is such a simple procedure that it is amazing that almost
no one knows how to do it. Yes, there are a few folks out there
who manage to obtain a tiny fraction of their mercury as sodium
amalgam. Usually, less than one percent. It does work but it can’t
work for very long before it is discharged and must be recharged.
Maybe there are a few of you who know much more than I do about
it. If so, please contact me. Charging
mercury is such a simple procedure that it is amazing that almost
no one knows how to do it. Yes, there are a few folks out there
who manage to obtain a tiny fraction of their mercury as sodium
amalgam. Usually, less than one percent. It does work but it can’t
work for very long before it is discharged and must be recharged.
Maybe there are a few of you who know much more than I do about
it. If so, please contact me.
 In
order to charge your mercury you must have a charging vessel. I
will be glad to sell such high tech vessels for only $29.99 plus
postage. Also I will sell you the necessary "charging salts"
for the amazing price of only $9.99 plus postage. For
those of you who insist on making your own I will provide
instructions free of charge. How’s that for a deal!
(I’m working on a kickback deal from your local supermarket). In
order to charge your mercury you must have a charging vessel. I
will be glad to sell such high tech vessels for only $29.99 plus
postage. Also I will sell you the necessary "charging salts"
for the amazing price of only $9.99 plus postage. For
those of you who insist on making your own I will provide
instructions free of charge. How’s that for a deal!
(I’m working on a kickback deal from your local supermarket).
 It will
require a trip to the super. You should purchase an "Old
Fashioned" tumbler glass of the hard, clear, plastic type.
Don’t try to get around me by using one of those soft,
polyethylene "tupperware" types. They won’t work.
In order to save a trip back to the store, buy a container of good
ol Red Devil Lye. You now have 90% of your materials in hand. If
you don’t have any epoxy glue, you should pick up a tube of
that too. It will
require a trip to the super. You should purchase an "Old
Fashioned" tumbler glass of the hard, clear, plastic type.
Don’t try to get around me by using one of those soft,
polyethylene "tupperware" types. They won’t work.
In order to save a trip back to the store, buy a container of good
ol Red Devil Lye. You now have 90% of your materials in hand. If
you don’t have any epoxy glue, you should pick up a tube of
that too.
 Now you
should drill or melt a small hole in the glass at the very bottom
of a size such that a solid copper wire of size # 14 or so can be
inserted in the hole and extended to the opposite side of the
glass. Seal around the wire at the hole and glue the loose end of
the wire the bottom of the glass. This will be your reaction
vessel with the cathode or negative terminal. You now must make an
anode or positive electrode. This can be piece of re-bar, a steel
bolt, an old screwdriver or whatever. I recommend using a bolt
with two nuts and a disc of steel a bit smaller than the vessel.
Cut a hole in the center so that it can be sandwiched between the
nuts. While this is not absolutely necessary, It will allow a bit
more current to flow through the cell with a resulting faster
charge rate. Now fashion an anode clamp to hold the anode and
prevent it from falling into the mercury. A piece of wood with a
tight fitting hole works fine. Now you
should drill or melt a small hole in the glass at the very bottom
of a size such that a solid copper wire of size # 14 or so can be
inserted in the hole and extended to the opposite side of the
glass. Seal around the wire at the hole and glue the loose end of
the wire the bottom of the glass. This will be your reaction
vessel with the cathode or negative terminal. You now must make an
anode or positive electrode. This can be piece of re-bar, a steel
bolt, an old screwdriver or whatever. I recommend using a bolt
with two nuts and a disc of steel a bit smaller than the vessel.
Cut a hole in the center so that it can be sandwiched between the
nuts. While this is not absolutely necessary, It will allow a bit
more current to flow through the cell with a resulting faster
charge rate. Now fashion an anode clamp to hold the anode and
prevent it from falling into the mercury. A piece of wood with a
tight fitting hole works fine.
 The
following drawing illustrates whatthe vessel should look like. The
following drawing illustrates whatthe vessel should look like.
. |
The Charging vessel. |
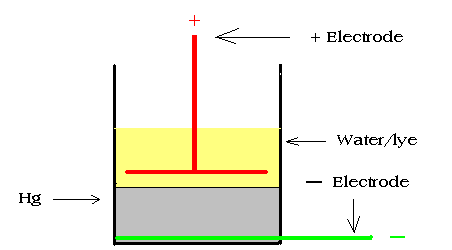
|
|
 Now all
you have to do is connect your reactor to a source of DC power
capable of delivering at least 1 ampere of current. A 12 volt car
battery is convenient for this purpose. You can use a battery
charger if you like. If you have two batteries you can connect
them in series and cut your charging time in half. You should
watch the system in the beginning just to be sure you are not
pulling too much current which will cause the cell to get too hot
and maybe boil. Don’t let it get that hot. This is not
usually a problem and is easily fixed by simply reducing the
amount of lye in the water layer or putting a light bulb in series
with the system. The amount of mercury in the cell and the amount
of current flowing through the system determine the time required.
You will know when the mercury is well charged because it will be
a gray, solid, putty-like mass. Not a liquid. At this point put on
gloves and pour off the water/lye layer, wash the mercury surface
with clean water and immediately dry it with an absorbent paper
towel. Store it in a clean, dry, tightly closed plastic bottle or
jar. Now all
you have to do is connect your reactor to a source of DC power
capable of delivering at least 1 ampere of current. A 12 volt car
battery is convenient for this purpose. You can use a battery
charger if you like. If you have two batteries you can connect
them in series and cut your charging time in half. You should
watch the system in the beginning just to be sure you are not
pulling too much current which will cause the cell to get too hot
and maybe boil. Don’t let it get that hot. This is not
usually a problem and is easily fixed by simply reducing the
amount of lye in the water layer or putting a light bulb in series
with the system. The amount of mercury in the cell and the amount
of current flowing through the system determine the time required.
You will know when the mercury is well charged because it will be
a gray, solid, putty-like mass. Not a liquid. At this point put on
gloves and pour off the water/lye layer, wash the mercury surface
with clean water and immediately dry it with an absorbent paper
towel. Store it in a clean, dry, tightly closed plastic bottle or
jar.
 Usually,
no matter how tight the bottle some water vapor will get in. You
can fix this problem by putting a packet of drying agent such as
silica gel, calcium sulfate, or calcium carbide in the container
with the mercury. These materials will effectively scrub out any
water, which gets in. Usually,
no matter how tight the bottle some water vapor will get in. You
can fix this problem by putting a packet of drying agent such as
silica gel, calcium sulfate, or calcium carbide in the container
with the mercury. These materials will effectively scrub out any
water, which gets in.
|
| A Few More Thoughts |
|
 I t
might be worth while discuss the subject of surface area as it of
paramount importance when amalgamating. First let me explain that
liquids always try attain a shape that results in the least
surface exposed. What shape would that be? Spherical. When
anything liquid or solid is in the shape of globe or sphere there
is no way to reduce the surface area more. If you change the shape
of a sphere to some other eg, A cube, a cylinder etc the surface
will increase to some extent. However, If you want to increase the
surface area by millions or hundreds of millions you simply divide
it into several separate pieces. If you take a marble and divide
it into 1000 smaller marbles the surface will increase by 100,000
times or so. Don’t hold me to these numbers they are only for
example. If you had divided it into 1000 cubes the area would have
been much more than the marble or the 1000 spheres. If you want to
increase it still further just grind it into finer and finer
particles. I think I recall reading that one lb of carbon ground
to face powder size or less would have a surface area more than
that of the entire earth. Something like that. I t
might be worth while discuss the subject of surface area as it of
paramount importance when amalgamating. First let me explain that
liquids always try attain a shape that results in the least
surface exposed. What shape would that be? Spherical. When
anything liquid or solid is in the shape of globe or sphere there
is no way to reduce the surface area more. If you change the shape
of a sphere to some other eg, A cube, a cylinder etc the surface
will increase to some extent. However, If you want to increase the
surface area by millions or hundreds of millions you simply divide
it into several separate pieces. If you take a marble and divide
it into 1000 smaller marbles the surface will increase by 100,000
times or so. Don’t hold me to these numbers they are only for
example. If you had divided it into 1000 cubes the area would have
been much more than the marble or the 1000 spheres. If you want to
increase it still further just grind it into finer and finer
particles. I think I recall reading that one lb of carbon ground
to face powder size or less would have a surface area more than
that of the entire earth. Something like that.
 Amalgamation
is very dependent upon surface area. Especially of the mercury. If
you keep your mercury in a single glob it will cause you less
headaches because there is less surface to corrode which can cause
"flouring". Also that single glob of mercury will take
much longer to contact all the gold particles. Amalgamation
is very dependent upon surface area. Especially of the mercury. If
you keep your mercury in a single glob it will cause you less
headaches because there is less surface to corrode which can cause
"flouring". Also that single glob of mercury will take
much longer to contact all the gold particles.
 If you
use charged mercury to recover gold from concentrates it’s
all right to allow it to break up into smaller globs. Things will
go much faster but you should try to get the mercury back in one
glob before it is discharged or the globs will corrode
rapidly and will be much more difficult to recover. If you
use charged mercury to recover gold from concentrates it’s
all right to allow it to break up into smaller globs. Things will
go much faster but you should try to get the mercury back in one
glob before it is discharged or the globs will corrode
rapidly and will be much more difficult to recover.
 So, the
more that charged mercury is spread out or its surface area
increased the more of the sodium is also spread out and the more
contact it has with the water. The more contact it has with water
the faster it reacts and the faster that it discharges. That is
bad for us. I wish I could say that you could use charged mercury
on a copper plate at the end of your sluice. Well, you can but it
won’t work very well. That little glob of charged mercury
that would last 30 minutes in your pan, when spread in a thin film
on a plate probably will last no longer than 30 seconds. So, the
more that charged mercury is spread out or its surface area
increased the more of the sodium is also spread out and the more
contact it has with the water. The more contact it has with water
the faster it reacts and the faster that it discharges. That is
bad for us. I wish I could say that you could use charged mercury
on a copper plate at the end of your sluice. Well, you can but it
won’t work very well. That little glob of charged mercury
that would last 30 minutes in your pan, when spread in a thin film
on a plate probably will last no longer than 30 seconds.
 I can
already hear some folks saying, "he ain’t so smart, I’ll
just take a battery and connect it right to the sluice plate and
keep the mercury charged all the time". I wish! It wont work
because if the mercury is not discharging its not a reducing agent
and will not clean either itself or the gold. It only works when
it is discharging. I can
already hear some folks saying, "he ain’t so smart, I’ll
just take a battery and connect it right to the sluice plate and
keep the mercury charged all the time". I wish! It wont work
because if the mercury is not discharging its not a reducing agent
and will not clean either itself or the gold. It only works when
it is discharging.
 I must
admit that I have sort of an idea how one might be able to use
charged mercury on a plate but like a lot of my ideas I haven’t
put it to the test yet. I must
admit that I have sort of an idea how one might be able to use
charged mercury on a plate but like a lot of my ideas I haven’t
put it to the test yet.
|
|
